Biopolymers have unique properties with respect to their self-assembly, conformational dynamics, transport and interactions with interfaces. Fluorescently labeled biopolymers and proteins can be studied by Quantitative Fluorescence Microscopy (QFM) and Fluorescence Correlations Spectroscopy (FCS).
We have studied the two-dimensional dynamics of DNA on supported lipid membranes using QFM and the conformational polymer dynamics of DNA in solution using FCS.One particular advantage of FCS is the fact that fluorescently labeled species can be followed in complex solution, e.g. solutions containing liposomes, blood proteins or full blood plasma. We used FCS to measure adsorption isotherms of peptides and proteins to liposomes.
Shear-Induced Unfolding and Enzymatic Cleavage of Full-Length VWF Multimers
S. Lippok, M. Radtke, T. Obser, L. Kleemeier, R. Schneppenheim, U. Budde, R. R. Netz, J. O. Rädler
Biophysical Journal (2016)
Proteolysis of the multimeric blood coagulation protein von Willebrand Factor (VWF) by ADAMTS13 is crucial for prevention of microvascular thrombosis. ADAMTS13 cleaves VWF within the mechanosensitive A2 domain, which is believed to open under shear flow. In this study, we combine fluorescence correlation spectroscopy (FCS) and a microfluidic shear cell to monitor real-time kinetics of full-length VWF proteolysis as a function of shear stress. For comparison, we also measure the Michaelis-Menten kinetics of ADAMTS13 cleavage of wild-type VWF in the absence of shear but partially denaturing conditions. Under shear, ADAMTS13 activity on full-length VWF arises without denaturing agent as evidenced by FCS and gel-based multimer analysis. In agreement with Brownian hydrodynamics simulations, we find a sigmoidal increase of the enzymatic rate as a function of shear at a threshold shear rate γ˙1/2 = 5522/s. The same flow-rate dependence of ADAMTS13 activity we also observe in blood plasma, which is relevant to predict hemostatic dysfunction.
Kinetics Models for Nanoparticles Interaction with Plasma Proteins
O. Vilanova, J. J. Mittag, P. M. Kelly, S. Milani, K. A. Dawson, J. O. Rädler and G. Franzese
ACS Nano (2016)
The interaction of nanoparticles with living cells is important with respect to both nanomedicine as well as nanotoxicity. We study various aspects of nanoparticles (NPs). The transport of NPs in biopolymer solutions, the adsorption of proteins to NPs, the uptake of NPs by cells and the interference of NPs with cellular signalling and cell death.
In a biological fluid, the surface of nanoparticles (NPs) is immediately modified by the adsorption of proteins, or other biomolecules, leading to the formation of a “protein corona”. This shell of biomolecules defines the real physicochemical properties of the nanoparticles: it determines the nanoparticles stability, and drives the uptake into the cells. Yet despite its role a comprehensive knowledge of the binding mechanisms and of the dependence of the protein corona on nanomaterial properties is still incomplete.
Here we use fluorescence correlation spectroscopy (FCS) to quantitatively analyse and model the adsorption of plasma proteins on different nanoparticle surfaces. Moreover, in order to understand the evolution of the protein corona as the nanoparticle moves from one biological environment to another, desorption kinetics in presence of competitive plasma proteins are also studied.
This work is funded by the EU-FP7 project: NanoTransKinetics
Size Distribution of the Mechanosensitive Blood Factor VWF
S. Lippok, T. Obser, J. P. Müller, V. K. Stierle, M. Benoit, U. Budde, R. Schneppenheim and J. O. Rädler
Biophysical Journal (2013)
Von Willebrand Factor (VWF) is a key protein in the force sensing cascade triggering primary hemostasis as it mediates the binding between thrombocytes and the injured vessel wall. VWF exists as multimers with variable number of dimeric subunits. Due to its shear flow sensitive structure, VWF functionality highly depends on its size. Although in recent years a general understanding of VWF function has emerged, its dynamic size regulation remains rather unexplored. We investigate the size distribution of recombinant eGFP-VWF using Fluorescence Correlation Spectroscopy (FCS). Following the size distribution of VWF in blood, we measure the in vivo and in vitro size regulation of VWF by the protease ADAMTS13, its most important size regulator. A two-focus FCS combined with a microfluidic device is established as a fast flow-through setup to investigate shear-induced changes of the VWF functionality.
This project is part of the DFG research unit FOR 1543 SHENC (Shear flow regulation of HEmostasis -bridging the gap between Nanomechanics and Clinical presentation) which is a collaboration between laboratories covering variable disciplines from medical research to theoretical biophysics: www.shenc.de
- C. Aponte-Santamaría, S. Lippok, J. J. Mittag, T. Obser, R. Schneppenheim, C. Baldauf, F. Gräter, U. Budde, J. O. Rädler
Mutation G1629E Increases von Willebrand Factor Cleavage via a Cooperative Destabilization Mechanism
Biophysical Journal, 112, (1), pp. 57–65, (2017) - S. Lippok, K. Kolšek, A. Löf, D. Eggert, W. Vanderlinden, J. P. Müller, G. König, T. Obser, K. Röhrs, S. Schneppenheim, U. Budde, C. Baldauf, C. Aponte-Santamaría, F. Gräter, R. Schneppenheim, J. O. Rädler, M. A. Brehm
von Willebrand factor is dimerized by protein disulfide isomerase
Blood (2016) - M. Radtke, S. Lippok, J. O. Rädler, R. R. Netz
Internal tension in a collapsed polymer under shear flow and the connection to enzymatic cleavage of von Willebrand factor
The European Physical Journal E (2016)
Adsorption Isotherms of Peptides and Proteins to Liposomes
- L. Rusu, A. Gambhir, S. McLaughlin and J. O. Rädler
Fluorescence Correlation Spectroscopy Studies of Peptide and Protein Binding to Phospholipid Vesicles
Biophysical Journal, 87 (2), 1044 - 1053, (2004) - Engelke, H., Lippok, S., Dorn, I., Netz, R. R., & Rädler, J. O.
FVIII Binding to PS Membranes Differs in the Activated and Non-Activated Form and Can Be Shielded by Annexin A5
The Journal of Physical Chemistry B, 115 (44), 12963 –12970, (2011)
Conformational Polymer Dynamics of DNA in Solution Measured by FCS
- Winkler, R., Keller, S., & Rädler, J.
Intramolecular dynamics of linear macromolecules by fluorescence correlation spectroscopy
Physical Review E, 73(4), (2006) - Lumma, D., Keller, S., Vilgis, T., & Rädler, J.
Dynamics of large semiflexible chains probed by fluorescence correlation spectroscopy
Physical Review Letters, 90(21), (2003)
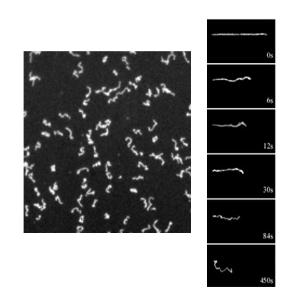
Two-dimensional Dynamics of DNA on Supported Lipid Membranes
- Maier, B., & Rädler, J.
Conformation and self-diffusion of single DNA molecules confined to two dimensions
Physical Review Letters, 82(9), 1911–1914, (1999) - Maier, B., & Rädler, J.
DNA on fluid membranes: A model polymer in two dimensions
Macromolecules, 33(19), 7185–7194, (2000) - Maier, B., & Rädler, J.
Shape of self-avoiding walks in two dimensions
Macromolecules, 34(16), 5723–5724, (2001) - Maier, B., Seifert, U., & Rädler, J.
Elastic response of DNA to external electric fields in two dimensions
Europhysics Letters, 60 (4), 622–628, (2002)
