Optical near-field microscopy
Utilizing scattering scanning near-field optical microscopy (s-SNOM) for mapping of nanostructures and spectroscopic investigation of biomaterials at the nanoscale
Utilizing scattering scanning near-field optical microscopy (s-SNOM) for mapping of nanostructures and spectroscopic investigation of biomaterials at the nanoscale
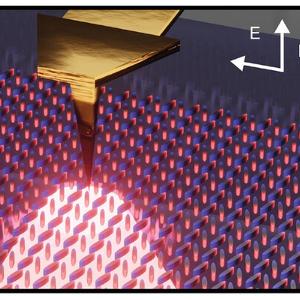
Scattering scanning near-field optical microscopy (s-SNOM) is a microscopy technique which allows to overcome the diffraction limit, one of the biggest constraints in optics, by nano-focusing light onto a sharp AFM tip. This creates a strongly confined near-field at the apex which interacts with the sample below, achieving spatial resolutions down to 20 nm. S-SNOM can be utilized both in the domains of photonics and solid state physics to resolve subwavelength features of e.g. polaritonic structures and nanoresonators, as well as for biospectroscopy, where it can be used to observe and image nanoparticles or living cells in both dry and aqueous environments, in addition to dynamically tracking spectroscopic changes over time.
We explore quasi-BIC metasurfaces using s-SNOM and discover that the quasi-BIC mode forms in just 10 × 10-unit cells, smaller than anticipated. We also find that resonator coupling, defects, and edge states significantly affect the mode. Our findings offer key insights for optimizing metasurface applications Adv. Mater. 2405978 (2024).
We utilize scattering-type scanning near-field optical microscopy (s-SNOM) for non-destructive, label-free mid-infrared (MIR) imaging and spectroscopy of photoswitchable liposomes, achieving sub-diffraction limit resolution and dynamic tracking down to 50 ms. We demonstrate the ability to observe photoinduced changes in both shape and MIR spectral signatures of individual vesicles, revealing abrupt change dynamics in the photoisomerization process. Our findings underscore the potential of this method for studying the complex dynamics of unlabeled nanoscale soft matter and broader applications in nanomedicine and material science arXiv.2406.02513 (2024).
We report that the tapping AFM tip in scattering scanning near-field optical microscopy (s-SNOM) causes a reversible nanometric deformation of ultrathin membranes, which depends on the driving force of the tapping cantilever. By minimizing these deformations, we enhance optical measurements and use the tapping phase delay as a sensitive indicator for studying the mechanics of adhering objects. This technique, which correlates mechanical responses with spectroscopy data, shows potential for depth profiling and studying mechano-active biopolymers and living cells under mechanical load Small, 2402568 (2024).
We demonstrate infrared scattering-type scanning near-field optical microscopy (s-SNOM) of water-suspended objects using a 10-nm thick SiN membrane, enabling nano-imaging of living cells in aqueous environments. This method allows us to observe living E. coli and A549 cancer cells at 150 nm resolution, revealing their structure, dynamics, and chemical composition by analyzing local abundances of water, proteins, and lipids. This SiN-membrane-based s-SNOM technique offers a powerful new tool for nanoscale analysis in various aqueous systems, from biology to materials science Sci Rep 11, 21860 (2021).
Take a look at the full list of publications here.