Lead halide nanocrystals have immense potential as tunable light emitters for displays, lighting, and light harvesting. Different synthesis methods yield nanoparticles with varying sizes, shapes, and compositions. Here, we aim to understand the mechanisms that determine size and shape. Our previous work (Nature Communications 2024) suggests that the rapid synthesis proceeds non-classically, involving several poorly understood steps:
1) Formation of precursor micelles,
2) Nanocluster formation upon precursor mixing,
3) Nanocluster decomposition and nanocrystal growth,
4) Superstructure formation.
We will employ advanced in-situ X-ray scattering and optical spectroscopy to resolve what happens on the atomic scale during synthesis. These experiments are conducted in collaboration with the group of Prof. Alex Urban (LMU Nanoinstitute), an expert in nanospectroscopy.
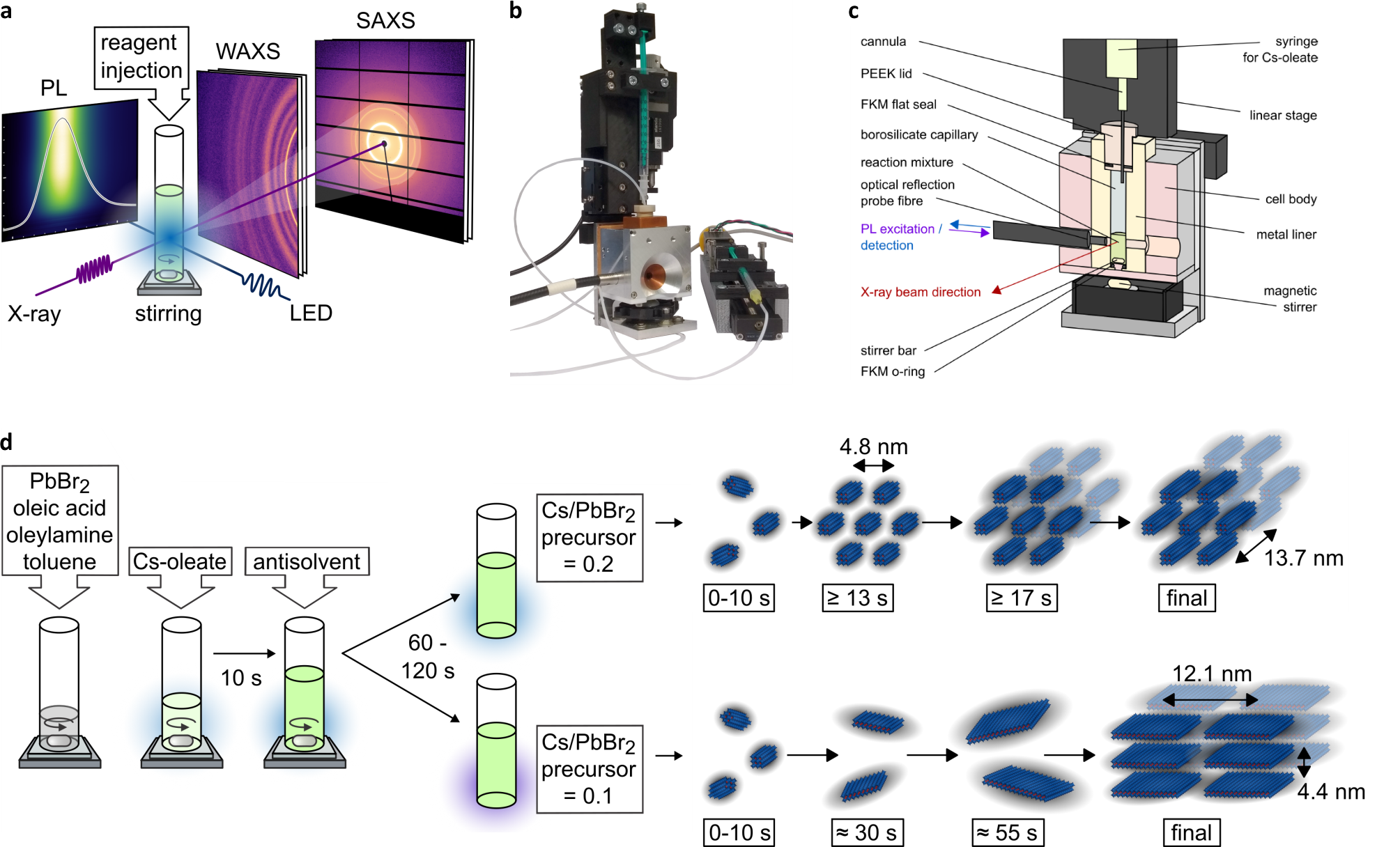
The image shows several aspects of quantum dot syntesis and their characterization by x-rays.
(a) Quantum dot synthesis takes place in a quartz glass vial. During the synthesis, we measure photoluminescence (PL) as well as the x-ray SAXS and WAXS signals.
(b) The reactants are injected into the vial remotely using two syringes controlled by a linear motor.
(c) The glass vial is embedded in a container specifically designed for in-situ x-ray experiments.
(d) The sequential injection of the reactants is carefully timed to optimize the quality of the quantum dots. Depending on the injection of a so-called antisolvent, quantum dots form as either nanorods or nanoplatelets.